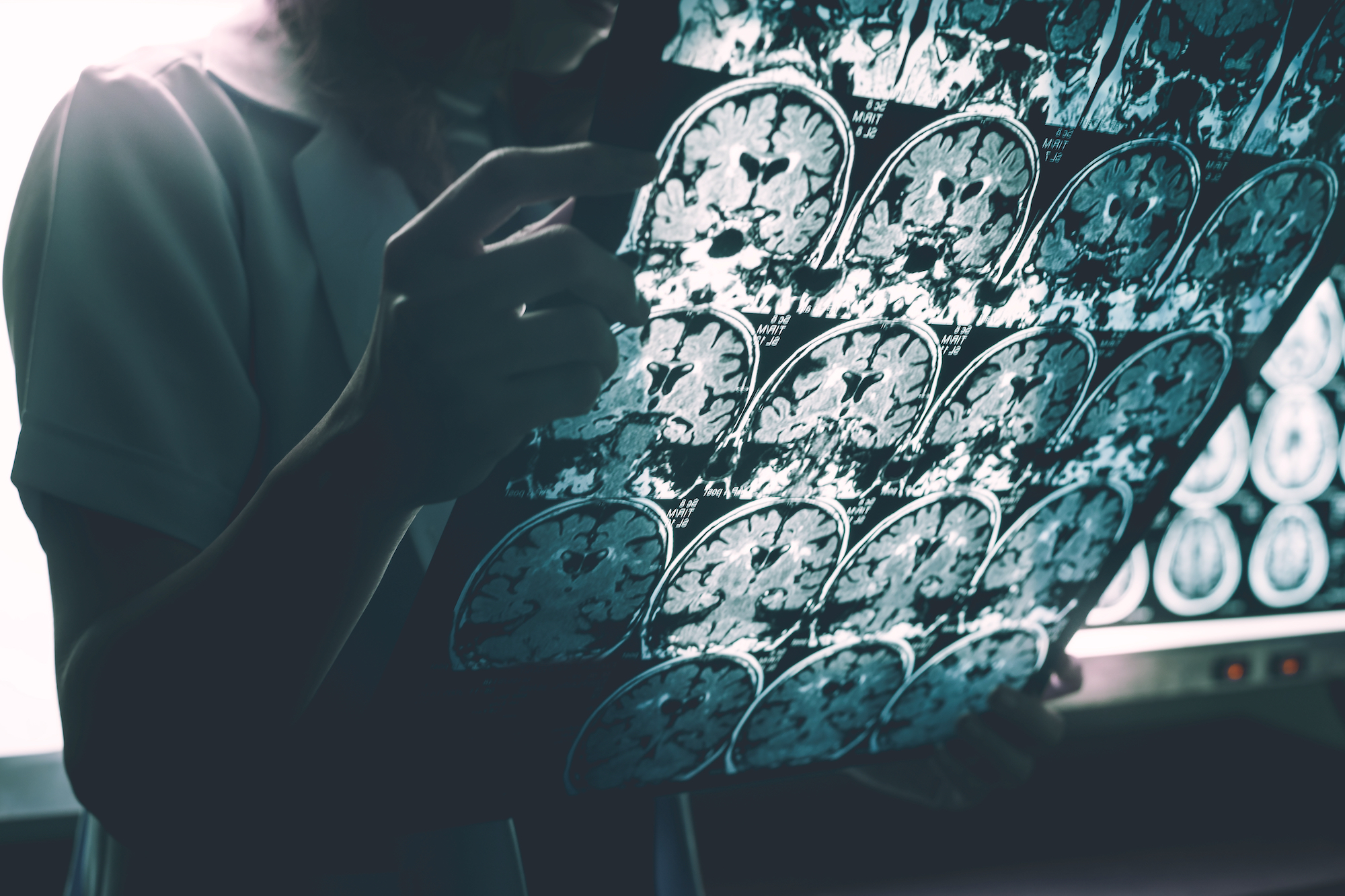
Research into the disease that afflicts in excess of 10.7 per cent of people aged 65 and over globally has primarily focused on plaques in the brain, but some scientists think viruses and bacteria play a role – and their work is gaining ground.

As Davangere Devanand, a neurologist at Columbia University Medical Centre, combed through the reams of scientific data on Alzheimer’s, he stumbled across a surprising idea – could an infection be involved in driving the disease?
“I was looking for an Alzheimer’s treatment approach that had a reasonable shot of working,” he says. “I found this old theory, going back 35 years, which linked herpes viruses to the disease, and there were all these indirect lines of evidence.”
The further Devanand looked, the more he found. Since the mid-80s, a handful of scientists around the world had doggedly pursued the idea that either a virus or a bacterium could play a role in Alzheimer’s, despite almost complete antipathy from those studying more accepted theories about the disease. Colleagues snubbed them, leading scientific journals and conferences rejected their work and funding had been threadbare, but slowly and surely, they built an increasingly compelling case.
In particular, evidence pointed towards herpes simplex virus 1 (HSV-1) – a pathogen found in three quarters of Australian adults, and the cause of oral herpes – as a prominent suspect. Studies in the UK, France and Scandinavia suggested that people who had been infected with herpes were more likely to get Alzheimer’s.
When Prof Ruth Itzhaki from Oxford University’s Institute of Population Ageing – who has done more than any other scientist to advance the HSV-1 theory of Alzheimer’s – examined postmortem brain samples from patients, she found greater amounts of the virus’s DNA than in people who had not died of the disease.
“Then there was this 2018 study from Taiwan, which was quite dramatic,” said Devanand. “When people with herpes were treated with a standard antiviral drug, it decreased their risk of dementia nine-fold.”

Devanand was intrigued because while the major hallmarks of Alzheimer’s are well-known, we still have little idea about what triggers it. We know that toxic plaques and tangles form inside the brain, causing damaging inflammation and the death of brain cells. Certain genes and lifestyle factors such as loneliness, lack of exercise and poor diet can all increase the risk of developing Alzheimer’s, but how and why it begins remains a mystery. Could a virus be the smoking gun that scientists have been looking for?
Others have suggested that various bacteria may also be capable of initiating the neurodegeneration that leads to Alzheimer’s. Chlamydia pneumoniae, which causes lung disease, Borrelia burgdorferi, which is associated with Lyme disease, and even gum infections have all been put forward as possible triggers.
The main idea for why viruses like HSV-1 and possibly bacteria may be capable of triggering Alzheimer’s is that they invade the body before burrowing into the central nervous system and travelling to the brain sometime in midlife. Once there, they stay dormant for many years before being reactivated in old age, either because the ageing immune system can no longer keep them in check, or something else – a traumatic episode, a head injury or perhaps another infection – spurs them to life. Once awakened – so the theory goes – they begin to wreak havoc.
For a long time, neurologists treated these ideas as fanciful, until more and more irrefutable evidence arose for the role of pathogens in chronic illness. Last year, the Epstein-Barr virus was identified as the main risk factor for multiple sclerosis, while other studies have shown that a bout of measles can lead many years later to a progressive neurological disorder called subacute sclerosing panencephalitis.

Based on the latest developments, the US National Institute on Aging agreed to run a clinical trial investigating whether a herpes antiviral drug called valacyclovir could slow the progression of Alzheimer’s in patients in the early stages of the disease. The ongoing trial, which is expected to be completed by early 2024, could have significant implications for how we look at the disease.
For decades, the number-one focus of almost all Alzheimer’s endeavours has been a protein fragment called beta-amyloid, often referred to as simply amyloid. Orthodox Alzheimer’s theories suggest that this accumulates in the brain as a kind of toxic waste, causing the signature plaques that kill brain cells and lead to the disease. Funding bodies and drug developers have largely shunned alternative explanations for why Alzheimer’s may occur, and instead have continued to pump resources into amyloid research.
But scientists are starting to show that the amyloid and microbial theories of Alzheimer’s may not be mutually exclusive. For while amyloid has long been seen as the villain of the story, some scientists believe it is actually a key element of our brain’s defence mechanisms against external threats.
Fifteen years ago, Rudolph Tanzi, a neurology professor at Harvard Medical School who has discovered many of the key genes linked to Alzheimer’s, discovered amyloid has antimicrobial properties, helping to defend the brain against any invading pathogen. More than a decade’s worth of experiments later, he has developed a viable theory for why plaques form.
“When an infection attacks your brain, your first response is these little sticky peptides that bind to the microbe, glutinate it into a ball and trap it,” he says. “We found that amyloid is one of the major peptides in the brain that goes after microbes. I believe the plaques we see in Alzheimer’s brains actually evolved as a way to protect the brain.”
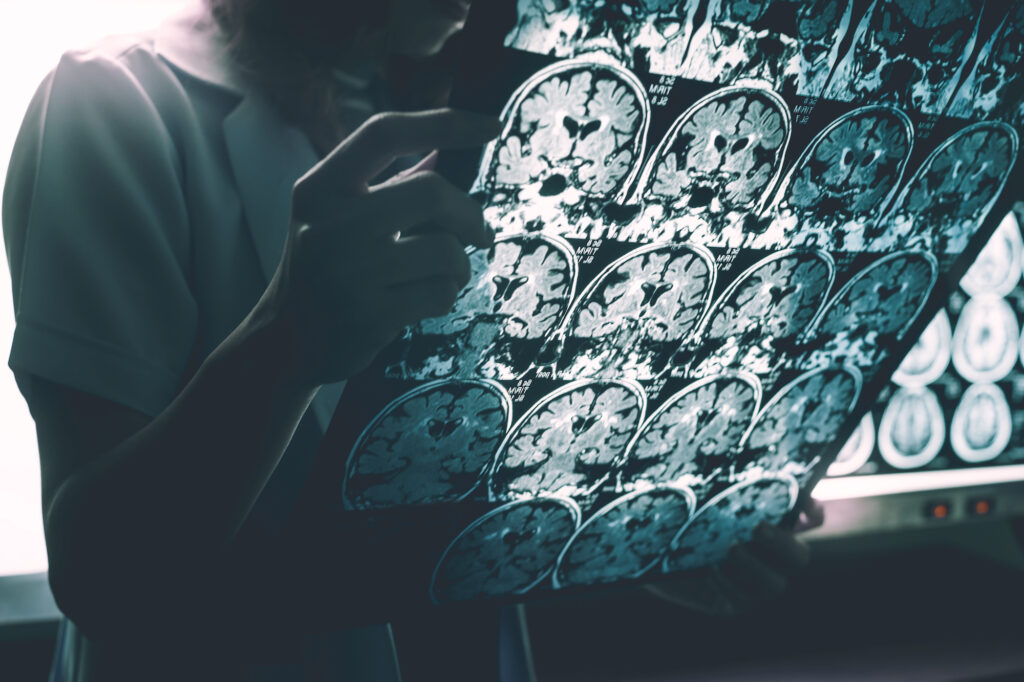
According to Tanzi, for much of our lives our body is able to seamlessly clear these clumps of amyloid. Immune cells known as microglia, which cleanse the brain of debris, gobble them up during deep sleep. But as we age, this finely tuned system can break down, and if amyloid is left lingering in the brain, it ends up harming us.
People with certain genetic vulnerabilities are unable to shovel out amyloid quite as efficiently as others, meaning it is more likely to accumulate. Ageing also weakens the immune system, making it easier for pathogens to access the brain and more amyloid to form.
“As you get older, your immune system starts to wane, and the barrier between your bloodstream and the brain is not what it used to be,” says Tanzi. “So you’re creating a perfect storm because microbes can proliferate better, and the brain does not clear amyloid as well as it used to.”
Hugo Lövheim, a researcher in geriatric medicine at Umeå University, says that we also know that lifestyle factors such as social isolation and lack of exercise can weaken the immune system. He suggests this could have two consequences – making it harder for the body to keep microbes such as HSV-1 in check, and then being unable to clear the resulting plaques.
The burden of Alzheimer’s and all forms of dementia on patients, families and society at large is unspeakably huge. In 2022 there were 401,300 Australians living with dementia, numbers that our healthcare system is not equipped to deal with. The cost of Alzheimer’s disease in this country is expected to rise by more than 70 per cent to around $26.6 billion over the next 20 years, but the starkest statistic is that two thirds of this burden is being covered by families themselves, either in unpaid care or private social care.
Some scientists suspect that the reason why anti-amyloid drugs have been ineffective in stopping Alzheimer’s is because we give them too late in the disease course, years or decades after the plaques have started to accumulate. By this point, it is widespread neuroinflammation that is killing off cells.
While anti-amyloid drugs could be tried on people in midlife to see if it stops them developing the disease, Tanzi says that the costs of this are likely to be impractical. Instead, if scientists can generate some more proof that a microbe is definitely instigating the disease in at least a proportion of patients, it could open the door to some more practical disease prevention initiatives. Making antivirals available to all people who have been infected by herpes could be one idea, or even encouraging more midlife vaccinations, for example against the varicella zoster virus (VZV) that causes shingles.
But Alzheimer’s is a devilishly complex disease and there is still much to be done in order to convince the majority of scientists that infections are involved. For while epidemiology and lab studies seem to show that it is possible, several clinical trials have ended in failure. It is hoped that the latest trial will provide crucial evidence regarding where to go next. If it shows some sign of benefit, it may help persuade funding bodies to stump up money to give antivirals to people in their 40s and 50s who are genetically at risk of Alzheimer’s or vaccinate a large number of people against various common viruses.
While Devanand, and a number of other scientists have dedicated their lives to this line of research, are hoping for just some hint that they are on the right track. “We’re not curing the disease in this trial,” he says. “We’re looking to see if patients who get an antiviral experience less decline than those who get a placebo. And if herpes is a contributing factor, it should be good to treat it.”
David Cox, freelance health journalist and former neuroscientist